Title : FDA Approves Apadaz (benzhydrocodone and acetaminophen) for the Short-Term Management of Acute Pain
link : FDA Approves Apadaz (benzhydrocodone and acetaminophen) for the Short-Term Management of Acute Pain
FDA Approves Apadaz (benzhydrocodone and acetaminophen) for the Short-Term Management of Acute Pain
In continuation of my update on Apadaz
KemPharm, Inc. announced the FDA approval of its New Drug Application (NDA) for Apadaz for the short-term (no more than 14 days) management of acute pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate. Apadaz is an immediate release (IR) combination of KemPharm’s prodrug, benzhydrocodone, and acetaminophen (APAP).
KemPharm, Inc. announced the FDA approval of its New Drug Application (NDA) for Apadaz for the short-term (no more than 14 days) management of acute pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate. Apadaz is an immediate release (IR) combination of KemPharm’s prodrug, benzhydrocodone, and acetaminophen (APAP).
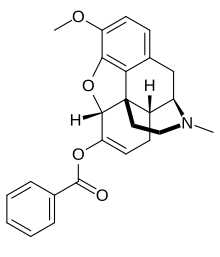 benzhydrocodone
benzhydrocodone “The approval of Apadaz is a significant milestone for KemPharm as it creates the opportunity to introduce what we believe is a differentiated product for the short-term management of acute pain,” said Travis Mickle, Ph.D., KemPharm President and Chief Executive Officer. “Based on its unique properties, we firmly believe there is a commercial pathway for Apadaz in what is a very high-volume market. We are excited by the opportunity Apadaz offers to patients and for physicians who now have the option of prescribing a differentiated product.”
“In addition to today’s approval, the U.S. Drug Enforcement Administration (DEA) has indicated that it is their intent to schedule Apadaz as a C-II product and will provide an allocation of the Active Pharmaceutical Ingredient (API) consistent with those scheduling provisions,” added Dr. Mickle. “This prompt decision by the DEA essentially completes the regulatory process with both Agencies and allows us to shift our focus towards the product launch.”
Thus Article FDA Approves Apadaz (benzhydrocodone and acetaminophen) for the Short-Term Management of Acute Pain
That's an article FDA Approves Apadaz (benzhydrocodone and acetaminophen) for the Short-Term Management of Acute Pain This time, hopefully can give benefits to all of you. well, see you in posting other articles.
You are now reading the article FDA Approves Apadaz (benzhydrocodone and acetaminophen) for the Short-Term Management of Acute Pain with the link address https://letslifes.blogspot.com/2018/03/fda-approves-apadaz-benzhydrocodone-and.html
0 Response to "FDA Approves Apadaz (benzhydrocodone and acetaminophen) for the Short-Term Management of Acute Pain"
Post a Comment