Title : Sun Pharma Announces FDA Approval of Yonsa (abiraterone acetate) to Treat Metastatic Castration-Resistant Prostate Cancer
link : Sun Pharma Announces FDA Approval of Yonsa (abiraterone acetate) to Treat Metastatic Castration-Resistant Prostate Cancer
Sun Pharma Announces FDA Approval of Yonsa (abiraterone acetate) to Treat Metastatic Castration-Resistant Prostate Cancer
In continuation of my update on abiraterone acetate
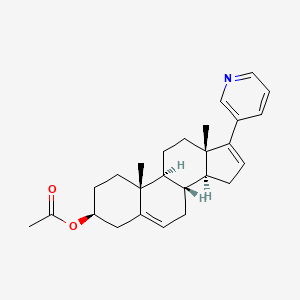
Sun Pharmaceutical Industries Ltd. and includes its subsidiaries and/or associate companies) and Churchill Pharmaceuticals, LLC. (Churchill) announced that one of Sun Pharma’s wholly owned subsidiary companies has received approval from the U.S. Food and Drug Administration (FDA) for Yonsa (abiraterone acetate), a novel formulation in combination with methylprednisolone, for the treatment of patients with metastatic castration-resistant prostate cancer (mCRPC).
“We are pleased to add Yonsa to our growing oncology portfolio and continue to deliver on Sun Pharma’s commitment for enhanced patient access to innovative cancer therapies,” said Abhay Gandhi, CEO - North America, Sun Pharma.
Yonsa in combination with methylprednisolone was filed as a New Drug Application (NDA) under the 505(b)(2) regulatory pathway and will be promoted as a branded product in the U.S.
Thus Article Sun Pharma Announces FDA Approval of Yonsa (abiraterone acetate) to Treat Metastatic Castration-Resistant Prostate Cancer
That's an article Sun Pharma Announces FDA Approval of Yonsa (abiraterone acetate) to Treat Metastatic Castration-Resistant Prostate Cancer This time, hopefully can give benefits to all of you. well, see you in posting other articles.
You are now reading the article Sun Pharma Announces FDA Approval of Yonsa (abiraterone acetate) to Treat Metastatic Castration-Resistant Prostate Cancer with the link address https://letslifes.blogspot.com/2018/07/sun-pharma-announces-fda-approval-of.html
0 Response to "Sun Pharma Announces FDA Approval of Yonsa (abiraterone acetate) to Treat Metastatic Castration-Resistant Prostate Cancer"
Post a Comment