Title : FDA Approves Jornay PM (methylphenidate) Extended-Release Capsules for Attention Deficit Hyperactivity Disorder (ADHD)
link : FDA Approves Jornay PM (methylphenidate) Extended-Release Capsules for Attention Deficit Hyperactivity Disorder (ADHD)
FDA Approves Jornay PM (methylphenidate) Extended-Release Capsules for Attention Deficit Hyperactivity Disorder (ADHD)
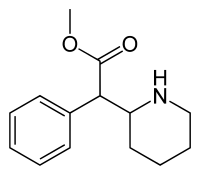
In continuation of my update on methylphenidate
Ironshore Pharmaceuticals & Development, Inc. (“Ironshore”) a wholly owned subsidiary of Highland Therapeutics Inc. (“Highland”) announced, that the U.S. Food and Drug Administration (FDA) has approved the New Drug Application (NDA) for Jornay PM (methylphenidate) (formerly known as HLD200) for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in patients 6 years and older. Jornay PM is a novel formulation of methylphenidate which is taken in the evening and has demonstrated improvement in the severity of ADHD symptoms in the early morning and throughout the day. Jornay PM is the first drug utilizing Ironshore’s proprietary drug delivery platform, Delexis®. Ironshore plans to initiate the commercial launch of Jornay PM in the first half of 2019.
According to independent research reports commissioned by Ironshore, control over the symptoms of ADHD during the early morning routine remains a significant concern for parents of children with ADHD. As previously reported in the Journal of Child and Adolescent Psychopharmacology, a majority of surveyed parents of children with ADHD report that the symptoms associated with ADHD in the early morning are described as “moderate” or “severe” during this time period.1
Commenting on the approval, David Lickrish, President & CEO stated, “Some manufacturers have adjusted the ratio of immediate-release and extended-release features in different formulations of methylphenidate to achieve an earlier onset. Our approach to drug development was to start from the desired pharmacokinetic profile and then work to develop a purpose-built technology capable of achieving that profile. I believe that the unique Delexis® drug delivery platform is a disruptive technology that has many applications and opportunities in several therapeutic categories.”
Delexis is a novel and proprietary drug delivery technology that contains two functional film coatings that act synergistically to achieve a unique pharmacokinetic profile. The first layer delays the initial release of drug for up to 10 hours while the second layer helps to control the rate of release of the active pharmaceutical ingredient throughout the day.
Dr. Bev Incledon, Head of Research and Development at Ironshore stated, “Developing a drug using a different delivery technology that will provide an additional option for patients and the physicians who treat them takes time. After 10 years of unrelenting determination, those efforts have finally been rewarded with the approval of Jornay PM. I want to thank and congratulate the many people who helped to make this possible including the formulation scientists, the Research & Development Team, the Investigators and the patients who participated in the clinical trials.”
The effectiveness of Jornay PM was established in two separate Pivotal Phase III, multicenter, randomized, double-blind, placebo-controlled studies conducted in a total of 278 pediatric patients aged 6 to 12 years with a diagnosis of ADHD per DSM-5 criteria. In addition to the traditional scales that assess efficacy in ADHD clinical trials such as the Swanson, Kotkin, Agler, M-Flynn, and Pelham (SKAMP) rating scale and the ADHD Rating Scale (ADHD-RS-IV), Ironshore’s pivotal trials assessed Jornay PM’s efficacy in the early morning period using the morning subscale of the Parent Rating of Evening and Morning Behavior-Revised (PREMB-R AM) scale and the Before School Functioning Questionnaire (BSFQ).
In Study 1, improvement in ADHD manifestations in a classroom setting was demonstrated by the primary endpoint, an average of all post-dosed SKAMP combined scores measured during a 12-hour period (8:00 a.m. to 8:00 p.m.), and improvement in ADHD manifestations in the early morning was demonstrated by the secondary endpoint, PREMB-R AM.
In Study 2, improvement in ADHD manifestations throughout the day was demonstrated by the primary endpoint, ADHD-RS-IV, and improvement in ADHD manifestations before school was demonstrated by the secondary endpoint, the BSFQ, which is intended to assess early morning before school activities from the time the child awakens and some behaviors not specific to early morning.
Commenting on the approval, Dr. Randy Sallee, Chief Medical Officer at Ironshore stated, “Many parents of children with ADHD note that the early morning routine is often one of the most chaotic times of the day. The idea of dosing the medication the night before was our moon-shot solution to meeting this need. The approval of Jornay PM is a welcome treatment option for healthcare providers, patients and their caregivers that may affect the way physicians think about ADHD treatment going forward.”
https://ift.tt/1PtOeG6
FDA Approves Jornay PM (methylphenidate) Extended-Release Capsules for Attention Deficit Hyperactivity Disorder (ADHD)
Thus Article FDA Approves Jornay PM (methylphenidate) Extended-Release Capsules for Attention Deficit Hyperactivity Disorder (ADHD)
That's an article FDA Approves Jornay PM (methylphenidate) Extended-Release Capsules for Attention Deficit Hyperactivity Disorder (ADHD) This time, hopefully can give benefits to all of you. well, see you in posting other articles.
You are now reading the article FDA Approves Jornay PM (methylphenidate) Extended-Release Capsules for Attention Deficit Hyperactivity Disorder (ADHD) with the link address https://letslifes.blogspot.com/2018/10/fda-approves-jornay-pm-methylphenidate.html
0 Response to "FDA Approves Jornay PM (methylphenidate) Extended-Release Capsules for Attention Deficit Hyperactivity Disorder (ADHD)"
Post a Comment