Title : FDA Approves Merck’s Delstrigo (doravirine/lamivudine/tenofovir disoproxil fumarate) for the Treatment of HIV-1 in Appropriate Patients
link : FDA Approves Merck’s Delstrigo (doravirine/lamivudine/tenofovir disoproxil fumarate) for the Treatment of HIV-1 in Appropriate Patients
FDA Approves Merck’s Delstrigo (doravirine/lamivudine/tenofovir disoproxil fumarate) for the Treatment of HIV-1 in Appropriate Patients
In continuation of my update on Lamivudine and Tenofovir
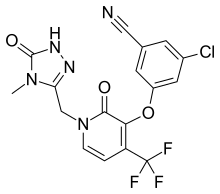
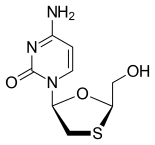
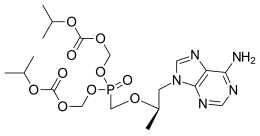
doravirine Lamivudine Tenofovir



doravirine Lamivudine Tenofovir
Merck known as MSD outside the United States and Canada, announced that the U.S. Food and Drug Administration (FDA) has approved Delstrigo, a once-daily fixed-dose combination tablet of doravirine (100 mg), lamivudine (3TC, 300 mg) and tenofovir disoproxil fumarate (TDF, 300 mg) for the treatment of HIV-1 infection in adult patients with no prior antiretroviral treatment experience. Delstrigo is administered orally once daily with or without food. Delstrigo contains a boxed warning regarding post-treatment acute exacerbation of hepatitis B (HBV) infection. Delstrigo does not cure HIV-1 infection or AIDS.
The FDA also approved Pifeltro (doravirine, 100 mg), the new non-nucleoside reverse transcriptase inhibitor (NNRTI) contained in Delstrigo, for administration in combination with other antiretroviral medicines.
Delstrigo is contraindicated when co-administered with drugs that are strong cytochrome P450 (CYP)3A enzyme inducers as significant decreases in doravirine plasma concentrations may occur, which may decrease the effectiveness of Delstrigo. Delstrigo is contraindicated in patients with a previous hypersensitivity reaction to 3TC. For more information, see “Selected Safety Information” below.
Immune reconstitution syndrome can occur, including the occurrence of autoimmune disorders with variable time to onset, which may necessitate further evaluation and treatment. Renal impairment, including cases of acute renal failure and Fanconi syndrome, have been reported with the use of TDF. Delstrigo should be avoided with concurrent or recent use of a nephrotoxic agent, as cases of acute renal failure after initiation of high-dose or multiple non-steroidal anti-inflammatory drugs (NSAIDs) have been reported in patients with risk factors for renal dysfunction who appeared stable on TDF.
Data Supporting the Approval of Delstrigo (doravirine 100 mg/3TC 300 mg/TDF 300 mg)
The FDA approvals of Delstrigo and Pifeltro are based on findings from the pivotal, randomized, multicenter, double-blind, active controlled Phase 3 trials, DRIVE-AHEAD and DRIVE-FORWARD, evaluating the efficacy and safety of Delstrigo and Pifeltro, respectively, in participants infected with HIV-1 with no antiretroviral treatment history.
Thus Article FDA Approves Merck’s Delstrigo (doravirine/lamivudine/tenofovir disoproxil fumarate) for the Treatment of HIV-1 in Appropriate Patients
That's an article FDA Approves Merck’s Delstrigo (doravirine/lamivudine/tenofovir disoproxil fumarate) for the Treatment of HIV-1 in Appropriate Patients This time, hopefully can give benefits to all of you. well, see you in posting other articles.
You are now reading the article FDA Approves Merck’s Delstrigo (doravirine/lamivudine/tenofovir disoproxil fumarate) for the Treatment of HIV-1 in Appropriate Patients with the link address https://letslifes.blogspot.com/2018/11/fda-approves-mercks-delstrigo_14.html
0 Response to "FDA Approves Merck’s Delstrigo (doravirine/lamivudine/tenofovir disoproxil fumarate) for the Treatment of HIV-1 in Appropriate Patients"
Post a Comment