Title : FDA Approves Xelpros (latanoprost ophthalmic emulsion) to Treat Open-angle Glaucoma or Ocular Hypertension
link : FDA Approves Xelpros (latanoprost ophthalmic emulsion) to Treat Open-angle Glaucoma or Ocular Hypertension
FDA Approves Xelpros (latanoprost ophthalmic emulsion) to Treat Open-angle Glaucoma or Ocular Hypertension
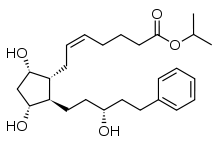
Sun Pharmaceutical Industries Ltd. and Sun Pharma Advanced Research Company Ltd. (SPARC) announced U.S. Food and Drug Administration (USFDA) approval for the New Drug Application (NDA) of Xelpros (latanoprost ophthalmic emulsion) 0.005% for the reduction of elevated intraocular pressure (IOP, or pressure inside the eye) in patients with open-angle glaucoma or ocular hypertension. This approval is from Sun Pharma’s Halol (Gujarat, India) facility.
Sun Pharma in-licensed Xelpros from SPARC in June 2015 and this approval will trigger a milestone payment to SPARC. SPARC is also eligible for milestone payments and royalties on commercialization of Xelpros in the US.
Xelpros is the first and only form of latanoprost that is not formulated with benzalkonium chloride (BAK), a preservative commonly used in topical ocular preparations. Xelpros is developed using SPARC’s proprietary Swollen Micelle Microemulsion (SMM) technology.
“As the only BAK-free version of latanoprost, Xelpros will be an important and alternative treatment option for individuals with open-angle glaucoma or ocular hypertension,” said Abhay Gandhi, CEO, North America, Sun Pharma. “This approval, coming less than one month following the approval of CEQUA™ (cyclosporine ophthalmic solution) 0.09%, reaffirms the strength of Sun Pharma’s fast-growing Ophthalmics division and its commitment to serving the needs of patients with ocular disorders.”
Anil Raghavan, CEO, SPARC said, “Approval of Xelpros by USFDA is a significant milestone for SPARC. It is also a validation of our SMM technology which helps to solubilize drugs that have limited or no solubility thus eliminating the need for benzalkonium chloride (BAK).”
In randomized, controlled clinical trials of patients with open-angle glaucoma or ocular hypertension with a mean baseline Intraocular pressure (IOP) of 23-26 mmHg, Xelpros lowered IOP by a mean of up to 6-8 mmHg.
Xelpros will be commercialized in the U.S. by Sun Ophthalmics, the branded ophthalmic division of Sun Pharmaceutical Industries Ltd.’s wholly owned subsidiary.
FDA Approves Xelpros (latanoprost ophthalmic emulsion) to Treat Open-angle Glaucoma or Ocular HypertensionThus Article FDA Approves Xelpros (latanoprost ophthalmic emulsion) to Treat Open-angle Glaucoma or Ocular Hypertension
That's an article FDA Approves Xelpros (latanoprost ophthalmic emulsion) to Treat Open-angle Glaucoma or Ocular Hypertension This time, hopefully can give benefits to all of you. well, see you in posting other articles.
You are now reading the article FDA Approves Xelpros (latanoprost ophthalmic emulsion) to Treat Open-angle Glaucoma or Ocular Hypertension with the link address https://letslifes.blogspot.com/2018/12/fda-approves-xelpros-latanoprost.html
0 Response to "FDA Approves Xelpros (latanoprost ophthalmic emulsion) to Treat Open-angle Glaucoma or Ocular Hypertension"
Post a Comment