Title : SK Life Science Announces FDA Acceptance of NDA Submission for Cenobamate, an Investigational Antiepileptic Drug
link : SK Life Science Announces FDA Acceptance of NDA Submission for Cenobamate, an Investigational Antiepileptic Drug
SK Life Science Announces FDA Acceptance of NDA Submission for Cenobamate, an Investigational Antiepileptic Drug
SK Life Science, Inc., a subsidiary of SK Biopharmaceuticals Co., Ltd., an innovative biopharmaceutical company focused on developing and bringing to market treatments for central nervous system (CNS) disorders, announced today that the U.S. Food and Drug Administration (FDA) has accepted the filing of its New Drug Application (NDA) for cenobamate. Cenobamate, an investigational antiepileptic drug for the potential treatment of partial-onset seizures in adult patients, is the first molecule discovered and developed from inception through to the submission of an NDA without partnering or out-licensing from a Korean pharmaceutical company. SK life science plans to commercialize cenobamate independently.
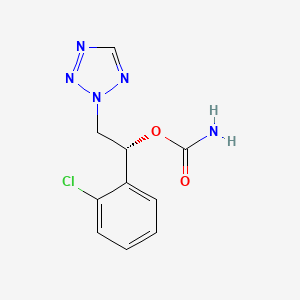
The NDA submission is based on data from pivotal trials that evaluated the efficacy and safety of cenobamate. Results from the clinical trial program, which enrolled more than 1,900 patients, have been presented at medical conferences including the American Academy of Neurology (AAN) and the American Epilepsy Society (AES) Annual Meetings.
"The FDA's acceptance of our NDA filing is a critical step toward our goal of introducing a new treatment option for people with uncontrolled epilepsy," said Marc Kamin, M.D., chief medical officer at SK life science. "We look forward to working with the FDA during their review of our data on cenobamate."
Despite the availability and introduction of many new AEDs, overall treatment outcomes for people with epilepsy have not improved in 20 years1 and the CDC states that nearly 60 percent of people with epilepsy are still experiencing seizures, showcasing a great unmet need for patients and their families.2Additionally, while some patients may experience a reduction in seizure frequency with current treatments, they continue to live with seizures.2 The impact of continued seizures can be debilitating and life-altering and the complications of epilepsy can include depression and anxiety, cognitive impairment and SUDEP (sudden unexpected death in epilepsy)
https://ift.tt/2EFAlH6
---------------------------------------------------------------------------------------------------------------
Thus Article SK Life Science Announces FDA Acceptance of NDA Submission for Cenobamate, an Investigational Antiepileptic Drug
That's an article SK Life Science Announces FDA Acceptance of NDA Submission for Cenobamate, an Investigational Antiepileptic Drug This time, hopefully can give benefits to all of you. well, see you in posting other articles.
You are now reading the article SK Life Science Announces FDA Acceptance of NDA Submission for Cenobamate, an Investigational Antiepileptic Drug with the link address https://letslifes.blogspot.com/2019/03/sk-life-science-announces-fda.html
0 Response to "SK Life Science Announces FDA Acceptance of NDA Submission for Cenobamate, an Investigational Antiepileptic Drug"
Post a Comment