Title : Karyopharm Announces FDA Extension of Review Period for Selinexor New Drug Application
link : Karyopharm Announces FDA Extension of Review Period for Selinexor New Drug Application
Karyopharm Announces FDA Extension of Review Period for Selinexor New Drug Application
Karyopharm Therapeutics Inc. (Nasdaq: KPTI), a clinical-stage pharmaceutical company, announced that the U.S. Food and Drug Administration (FDA) has extended the Prescription Drug User Fee Act (PDUFA) action date for the New Drug Application (NDA) for selinexor. The NDA, which is currently under Priority Review by the FDA, is seeking accelerated approval for selinexor in combination with dexamethasone for the treatment of patients with relapsed refractory multiple myeloma who have received at least three prior therapies and whose disease is refractory to at least one proteasome inhibitor (PI), one immunomodulatory agent (IMiD), and one anti-CD38 monoclonal antibody. The previously disclosed April 6, 2019 PDUFA date has been extended by three months to July 6, 2019.
On February 26, 2019, the FDA'sOncologic Drugs Advisory Committee (ODAC) met to discuss the selinexor NDA and voted 8 to 5 recommending that the FDA wait for the results from Karyopharm's randomized, open-label, Phase 3 BOSTON study evaluating selinexor in patients with relapsed or refractory multiple myeloma, before making a final decision regarding approval. Although the FDA considers the recommendation of this panel, the final decision regarding the approval of the product is made by the FDA solely, and the recommendations by the panel are non-binding.
Following the ODAC meeting, at the FDA's request, Karyopharm submitted additional, existing clinical information as an amendment to the NDA, which allowed the FDA to extend the PDUFA action date by three months. "We look forward to the continued collaboration with FDA in trying to meet the needs of patients with relapsed refractory multiple myeloma," said Sharon Shacham, PhD, MBA, Founder, President and Chief Scientific Officer of Karyopharm.
About Selinexor
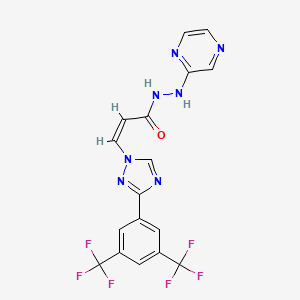
Selinexor is a first-in-class, oral Selective Inhibitor of Nuclear Export (SINE) compound. Selinexor functions by binding with and inhibiting the nuclear export protein XPO1 (also called CRM1), leading to the accumulation of tumor suppressor proteins in the cell nucleus. This reinitiates and amplifies their tumor suppressor function and is believed to lead to the selective induction of apoptosis in cancer cells, while largely sparing normal cells. In 2018, Karyopharm reported positive data from the Phase 2b STORM study evaluating selinexor in combination with low-dose dexamethasone in patients with triple class refractory multiple myeloma who have been previously exposed to all five of the most commonly prescribed anti-myeloma therapies currently available. Selinexor has been granted Orphan Drug Designation in multiple myeloma and Fast Track designation for the patient population evaluated in the STORM study. Karyopharm's New Drug Application (NDA) has been accepted for filing and granted Priority Review by the FDA, and oral selinexor is currently under review by the FDA as a possible new treatment for patients with triple class refractory multiple myeloma. The Company has also submitted a Marketing Authorization Application (MAA) to the European Medicines Agency (EMA) with a request for conditional approval and was granted accelerated assessment. Selinexor is also being studied in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL). In 2018, Karyopharm reported positive top-line results from the Phase 2b SADAL study evaluating selinexor in patients with relapsed or refractory DLBCL after at least two prior multi-agent therapies and who are ineligible for transplantation, including high dose chemotherapy with stem cell rescue. Selinexor has received Fast Track designation from the FDA for the patient population evaluated in the SADAL study. Selinexor is also being evaluated in several other mid-and later-phase clinical trials across multiple cancer indications, including in multiple myeloma in a pivotal, randomized Phase 3 study in combination with Velcade® (bortezomib) and low-dose dexamethasone (BOSTON), as a potential backbone therapy in combination with approved therapies (STOMP), in liposarcoma (SEAL), and an investigator-sponsored study in endometrial cancer (SIENDO), among others. Additional Phase 1, Phase 2 and Phase 3 studies are ongoing or currently planned, including multiple studies in combination with approved therapies in a variety of tumor types to further inform Karyopharm's clinical development priorities for selinexor. Additional clinical trial information for selinexor is available at www.clinicaltrials.gov.
http://bit.ly/2V937eh
Thus Article Karyopharm Announces FDA Extension of Review Period for Selinexor New Drug Application
That's an article Karyopharm Announces FDA Extension of Review Period for Selinexor New Drug Application This time, hopefully can give benefits to all of you. well, see you in posting other articles.
You are now reading the article Karyopharm Announces FDA Extension of Review Period for Selinexor New Drug Application with the link address https://letslifes.blogspot.com/2019/05/karyopharm-announces-fda-extension-of.html
0 Response to "Karyopharm Announces FDA Extension of Review Period for Selinexor New Drug Application"
Post a Comment